Pharmaceutical companies often argue that they need to charge high prices for their drugs in the...
Drugs and technology
Sanders bill would allow drug importation, lower costs
The way to ensure prescription drugs are affordable in the U.S. is for the government to establish...
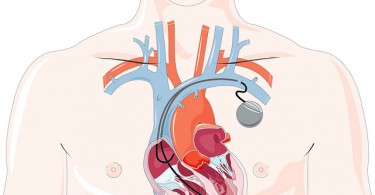
Can your pacemaker be hacked?
An article on Wired.com describes the scary security issues that come with high-tech medical...
Get engaged in the drug price fight!
Although it remains unclear whether Congress or the Trump administration will try to tackle the...
Link found between researchers’ financial ties to drug...
Financial ties to the pharmaceutical industry are common among researchers, guideline panelists...
What does a Trump FDA look like?
There are many reasons to be concerned about food and drug safety today. Even with an FDA committed...
Cures Act threatens drug safety and efficacy
After an intense lobbying campaign heavily funded by the pharmaceutical industry and featuring sick...
More than four in ten clinical trial results are not available...
To shed more light on safe and effective drug treatments, the U.S. Department of Health and Human...
Three reasons why a faith-based movement can help fix our broken...
When it comes to our medicines system, it is hard to overstate how profoundly broken it is. Here in...
Your tax dollars are making big Pharma rich–twice
Have you heard the old saying that, if you look around a poker table and can’t figure out who is...