Stat News reports on a new study, published in BMJ, which finds that around 50 percent of the clinical trials for cancer treatments approved in Europe may not demonstrate meaningful benefits. Risk of bias in the design and reporting of clinical trials may mean that the drugs lack both therapeutic and financial value. Studies of clinical trials for cancer drugs approved in the US have similar flaws.
For the BMJ study, researchers looked at the strength of the evidence supporting new cancer drug approvals in Europe. They analyzed 54 clinical trials for the 32 cancer drugs approved between 2014 and 2016. Of those with published studies, only one in four looked at overall survival rates. All the other trials, 29 out of 39, used “surrogate measures.” Surrogate measures cannot predict whether someone will live longer or enjoy a better quality of life. Some academics say they may undermine patient safety.
Researchers found that just about half (49 percent) of the published trials had a high risk of bias. They found a much higher risk of bias in the trials looking at surrogate measures of patient benefit (55 percent) than in the trials measuring overall survival (20 percent). Note: Researchers found risk of bias, not actual bias.
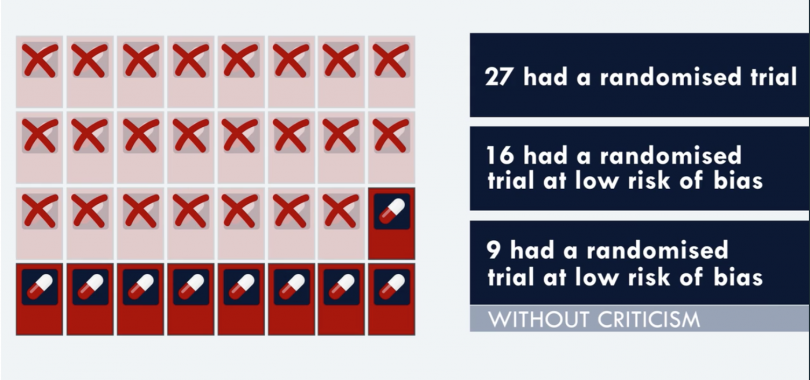
Only 75 percent of the drug studies (41 of them) from 2014 to 2016 involved randomized, controlled trials. Just 27 of the 32 newly approved cancer drugs had a randomized trial. Between, 2009 and 2013, 90 percent of the studies involved randomized, controlled trials.
In addition, of the 32 drugs approved, 10 had problems identified by regulators. But, somehow, the regulators’ concerns about these drugs did not make it into the scientific literature.
The researchers recommend that clinical research for drugs be based on randomized trials and that these trials study data on meaningful outcomes, specifically overall survival. Without this research, it is hard to know whether new cancer drugs meet patients’ needs. It’s best to have a quality assessment of the evidence as well. Bias in clinical trial design is a serious issue.
The researchers also want more transparency from regulators. Patients should know the weaknesses of the research underlying a new drug and how these weaknesses might affect the trial results.
Here’s more from Just Care:
- FDA approved medical devices may not be safe
- Majority of cancer drugs that FDA has recently approved don’t work
- One in three FDA-approved drugs have safety risks
- If you take supplements, beware of potentially serious supplement-drug interactions
- Five exercises to improve balance for safety and health